Abstract
Introduction
Selinexor is an exportin-1 (XPO1) inhibitor which forces the nuclear retention and functional activation of tumor suppressor proteins, inducing apoptosis in cancer cells. Overexpression of XPO-1 is common in many tumors, including acute myeloid leukemia (AML). A phase II study, "SAIL", of selinexor with cytarabine and idarubicin in patients with relapsed/refractory AML was conducted by Fiedler et al. showing a high remission rate. To identify molecular predictors of response and survival we evaluated the molecular mutations and their course in selinexor treated patients.
Methods
All 42 patients treated in the SAIL trial were eligible to participate in this translational study. The main criterion for inclusion in the present study was availability of DNA from bone marrow or peripheral blood at 3 time points: initial AML diagnosis, screening for the SAIL trial and first response assessment on day 28 of cycle 1. A custom TruSight myeloid sequencing panel was used to identify molecular mutations at diagnosis. Molecular response was defined as variant-allele frequency (VAF) <1% in the follow-up sample after SAIL treatment independent of morphologic remission. Minimal residual disease (MRD) under selinexor maintenance treatment was quantified by error-corrected NGS with a limit of detection of 0.02%.
Results
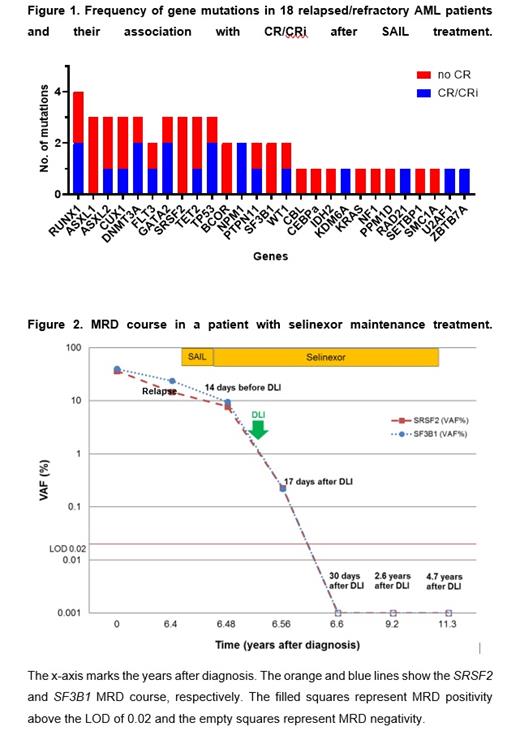
Eighteen patients were included for whom DNA was available for all three time points. The median age was 47.5 years (29-72), median prior therapies was 3 (1-9). 13 patients had de novo and 5 secondary/therapy-related AML. ELN risk at first diagnosis was favorable in eight, intermediate in five and adverse in three patients. Only one course of SAIL treatment was administered to all patients. 8 patients achieved morphologic complete remission (CR) or CR with incomplete hematologic recovery (CRi). 13 patients proceeded to allogeneic hematopoietic cell transplantation (alloHCT) or donor lymphocyte infusion. The median overall survival was 0.69 years. The molecular profile showed a predominance of secondary AML-type mutations. No clear pattern was found between mutation status and morphologic CR or CRi (Figure 1). Molecular response to the SAIL induction treatment was found in 6 of 14 patients who had a molecular marker. Mutations in FLT3 (FLT3-TKD=1, FLT3-ITD=2), SF3B1 and TP53 were associated with molecular response, whereas mutations in GATA2, CUX1, TET2, BCOR, DNMT3A, RAD21, ASXL1, SRSF2, and WT1 were associated with resistance. When comparing the molecular characteristics of patients achieving CR/CRi (n=8) and all other patients (n=10), a trend to achieve CR was observed among patients with NPM1 mutations (P=0.094), whereas mutations in ASXL1 (P=0.09) and SRSF2 (P=0.09) were associated with refractoriness.
One of the responding patients received selinexor as maintenance therapy for four years. The patient was diagnosed with de novo AML with normal cytogenetics, with SF3B1 and SRSF2 mutations. The patient received an HLA-identical transplant after myeloablative conditioning, but relapsed 6 years after alloHCT. One cycle of selinexor/chemotherapy was administered and the patient achieved CR. The patient continued selinexor maintenance treatment with 60 mg selinexor twice a week. SF3B1 and SRSF2 mutations were still present at the time of relapse and declined under SAIL treatment (Figure 2). The patient received one course of DLI (1x10 7CD3 +), which was tolerated well without signs of GvHD. MRD remained detectable 17 days after DLI. At 30 days after DLI treatment both MRD markers were negative. Under continued selinexor maintenance treatment MRD remained negative until last follow-up at 4.9 years after SAIL treatment. The patient tolerated selinexor well with short-term nausea and dysgeusia after selinexor intake. Selinexor maintenance treatment was stopped 4 years after SAIL treatment and the patient remains in CR 14 months after the end of maintenance.
Conclusion
In this small series, we found a correlation between FLT3, TP53 and SF3B1 mutation status and molecular response to selinexor/chemotherapy (SAIL). NPM1 mutations were associated with morphologic response to SAIL by trend. Finally, selinexor maintenance may have contributed to long-term disease control in a patient with relapsed AML, and long term therapy with selinexor is feasible.
Fiedler: Servier: Consultancy, Other: Meeting attendance, Preparation of information material; Stemline: Consultancy; Daiichi Sanyko: Consultancy, Other: Meeting attendance, Preparation of information material; Pfizer: Consultancy, Honoraria, Research Funding; Novartis: Honoraria; MorphoSys: Consultancy, Honoraria; Jazz: Consultancy, Honoraria, Other: Meeting attendance, Preparation of information material; Celgene: Consultancy, Honoraria; Ariad/Incyte: Honoraria; Amgen: Consultancy, Honoraria, Other: Meeting attendance, Preparation of information material, Patents & Royalties, Research Funding; Abbvie: Consultancy, Honoraria, Other: Meeting attendance, Preparation of information material. Modemann: Servier: Honoraria, Other: Travel accomodation; Incyte: Other: Travel accomodation; Gilead: Other: Travel accomodation; Jazz Pharmaceuticals: Other: Travel accomodation; Novartis: Other: Travel accomodation; Teva: Other: Travel accomodation; Pfizer: Other: Travel accomodation; Amgen: Other: Travel accomodation; Daiichi Sankyo: Research Funding; Abbvie: Honoraria, Other: Travel accomodation. Bokemeyer: Merck KGaA: Honoraria; Sanofi: Consultancy, Honoraria, Other: Travel accomodation; Roche: Honoraria, Research Funding; Bayer: Honoraria, Research Funding; BMS: Honoraria, Other: Travel accomodation, Research Funding; AstraZeneca: Honoraria, Research Funding; Merck Sharp Dohme: Consultancy, Honoraria; Lilly/ImClone: Consultancy; Merck Serono: Consultancy, Other: Travel accomodation ; Bayer Schering Pharma: Consultancy; GSO: Consultancy; AOK Health insurance: Consultancy; Abbvie: Research Funding; ADC Therapeutics: Research Funding; Agile Therapeutics: Research Funding; Alexion Pharmaceuticals: Research Funding; Amgen: Research Funding; Apellis Pharmaceuticals: Research Funding; Astellas: Research Funding; BerGenBio: Research Funding; Blueprint Medicine: Research Funding; Boehringer Ingelheim: Research Funding; Celgene: Research Funding; Daiichi Sankyo: Research Funding; Eisai: Research Funding; Gilead Sciences: Research Funding; Gylcotope GmbH: Research Funding; GlaxoSmithKline: Research Funding; Inside: Research Funding; IO Biotech: Research Funding; Isofol Medical: Research Funding; Janssen-Cilag: Research Funding; Karyopharm Therapeutics: Research Funding; Lilly: Research Funding; Millenium: Research Funding; MSD: Research Funding; Nektar: Research Funding; Rafael Pharmaceuticals: Research Funding; Springworks Therapeutics: Research Funding; Taiho Pharmaceutical: Research Funding; Pfizer: Other. Ganser: Celgene: Honoraria; Novartis: Honoraria; Jazz Pharmaceuticals: Honoraria. Thol: Astellas: Honoraria; Jazz: Honoraria; Novartis: Honoraria; Pfizer: Honoraria; BMS/Celgene: Honoraria, Research Funding; Abbvie: Honoraria. Heuser: Karyopharm: Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Tolremo: Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer Pharma AG: Research Funding; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; BergenBio: Research Funding; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Selinexor in patients with relapsed or refractory AML

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal